Seawater CO2
Seawater Program
As part of the Scripps CO2 Program's goal of understanding the global carbon cycle, the program's seawater program has been measuring the CO2 content of seawater since the late 1970's. Using high-precision methods for measuring total dissolved inorganic carbon (DIC), total alkalinity , and carbon and oxygen isotopes from CO2, the program has participated in numerous one-time surveys, maintains a number of time series stations, and serves as a calibration laboratory for a number of programs.
Dissolved Inorganic Carbon (DIC) Measurements
The carbon dissolved in sea water is generally divided into two major categories. Dissolved carbon derived from the decay of plants and animals as well as from their waste products, is referred to as dissolved organic carbon, or DOC. DOC mostly consists of long-chain organic molecules, but smaller species, such as methane, are also considered organic. The second form of dissolved carbon in sea water is dissolved inorganic carbon, or DIC. DIC in sea water consists of carbon in three forms: carbon dioxide (CO2), bicarbonate (HCO3), and carbonate (CO3). The relative abundance of these three carbon compounds is controlled by the pH of the water, while the total amount of DIC is controlled by a much more complicated set of factors.
Most origins of DIC are biological in nature, but the one we are interested in here at the Scripps CO2 program is anthropogenic. As the concentration of CO2 in our atmosphere increases, so does the amount of CO2 in the ocean's surface waters. Why is this important? While the oceans are able to remove some of the increasing carbon from the atmosphere, just how much they are able to remove has vital implications in determining the potential severity of global warming. Here at the Scripps CO2 program, our laboratory DIC measurements are accomplished in what is essentially a two step process.
Step 1: Vacuum Extraction
The first step is the physical extraction of the DIC from the liquid sea water. Our extraction procedure is relatively straight forward. In an evacuated extraction flask, we acidify an aliquot of sea water with dilute phosphoric acid, which converts all forms of inorganic carbon (dissolve carbonate and bicarbonate) into CO2 gas. The extraction flask is then opened to a vacuum line, and the gasses evolved (mostly water vapor, nitrogen, oxygen and CO2) are drawn through a series of cryogenic traps. First, water vapor is removed from the extracted stream of gasses with a dry ice/ethanol trap (about -90º C) and the CO2 is collected using a trap filled with liquid nitrogen (about -190º C). The remaining gasses do not freeze at the temperatures of the traps, and are therefore allowed to simply pass through the line and are pumped away.
Extraction Line Schematic:
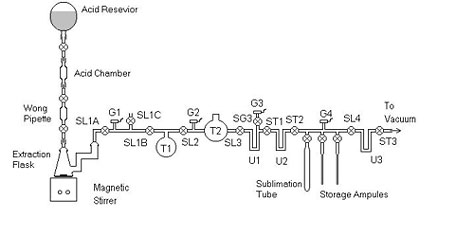
The aliquot of extracted CO2 is then further purified via sublimation using "plastic ethanol" (ethanol chilled to about -100º C) and cryogenically transferred into an ampule of about 1cc volume, which is sealed under vacuum and stored for later quantitative analysis.

Step 2: Manometric Analysis
After extraction, the CO2 ampules are gathered and run on our Electronic Constant-volume Manometer (ECM). This instrument is designed to measure the pressure of a small amount of gas very precisely. The pressure sensing device consists of a Ruska DDR6000 Quartz Spiral manometer and a Differential Pressure Indicator (DPI), which are mated to a confined volume of glass into which our samples are transferred at the time of measurement. The DPI and the volume are both contained in a constant temperature air bath which is kept at 40º C. The Quartz spiral itself is kept at a constant temperature in a chamber of it's own. The sample volume is calibrated by the introduction of a precisely defined amount of gas into the volume itself. The pressure produced by this gas is measured, and, using the non-ideal gas law, the volume of the chamber is calculated. These calibration gases are produced by filling small (1 to 2.5 cc) glass "plenums" with CO2 at a precisely known pressure supplied by a Ruska Dead Weight Tester. The volumes of the plenums themselves were determined by weighing the volumes filled with degassed water on a micro-balance.
The Scripps CO2 Program Electronic Constant-Volume Manometer:

The pressure measured for each unknown sea water is combined with the calibrated volume of the ECM, again using the non- ideal gas law, to arrive at the number of moles of CO2. This quantity is then divided by the weight of the aliquot of water from which this CO2 was extracted to give us the DIC content of the sample. To eliminate variations in concentration caused by short term dilution of the water mass due to precipitation/evaporation cycles, the raw DIC is often normalized to a constant salinity (salinity is quite a conservative property of sea water, meaning that is usually unaffected by anything other than precipitation/evaporation cycles).
Total Alkalinity Measurements
Total alkalinity is a measure of the amount of negatively charged ions in a given amount of water. In the oceans, most of the negatively charged ions present are bicarbonate (HCO3-) and carbonate (CO3--). Since the other ionic species which contribute to the amount of alkalinity in sea water are present in both small and relatively well known amounts, when we measure the alkalinity of a sea water sample, we are really measuring is the amount of these two major compounds.
Total alkalinity is important to us here at the Scripps CO2 Program because it, along with the amount of DIC in a batch of sea water, can be used to calculate the amount of dissolved carbon dioxide gas (pCO2) in that batch of sea water. Precise measurements of total alkalinity are thus vital to our efforts to understand how the oceans are responding to the increase of CO2 in the atmoshphere. We have traditionally used the closed-cell titration method to measure total alkalinity.
Closed Cell Alkalinity Titration
We measure the amount of total alkalinty in sea water by carrying out a potentiometric titration of a weighed aliquot of sea water in a vessel which is sealed from the atmosphere. This is accomplished by adding precise amounts of 0.1 normal hydrochloric acid (HCl) to the vessel in small increments, and measuring the change in the electromotive potential (emf) of the water caused by this addition. The potential of the sea water is measured with a standard combination-type pH electrode, and the whole cell is encased in a jacket through which constant temperature water is pumped. To ensure that the sample maintains a constant temperature (temperature has a direct effect on the emf of the water), an electronic temperature probe is also mounted in the cell.The entire procedure is run by a computer, which also monitors and records the response of the probes. Once the titration is complete, the data (acid volume, emf, and temperature) are used to calculate the total alkalinity by the modified gran method. While the math behind all this is rather complicated, what we are actually doing is determining how many protons (supplied by the HCl) were consumed in converting all the carbonate and bicarbonate in the sample to CO2.Looking at a plot of this titration data, it is easy to visualize just where this occurs. As the positively charged protons (H+) are added to the mix, they react with the carbonate (CO3--) to convert it to bicarbonate (HCO3-), causing the first of two inflection points on the titration curve. As more acid is added, all of the bicarbonate is converted to CO2, which causes the second inflection point on our curve. The location of this second inflection point is what determines the total alkalinity of the sample.
